Background
There are four types of states of matter: solid, liquid, gas, and plasma. Among them, “liquid” state, represented by water, has an incompressible volume but no distinct shape, unlike the other three states.
Observation of the movement of droplets on a solid surface has become important because features including flowability and surface tension are the basis of phenomena such as liquid wettability, infiltration property, and sliding property [1].
Scientists are working to elucidate this by observing the behavior of droplets on solid surfaces [2].
(As the phenomenon unravels, it will also solve some of the energy issues that have been addressed in the SDGs.)
As a matter of fact, it has been found that observing the epidermis of plants and animals in nature is very useful for understanding such things. In Principia Lab, we will explain the principles necessary to understand interface physics, while also introducing the surface structures of plants and animals.
Wettability (Young’s equation )
Wettability is a property that expresses the degree to which a water droplet on a solid’s surface will spread when wet.
As we dig deeper into this property, we can understand things such as “why the yogurt doesn’t stick to the lid of the yogurt package”.
In general, this property is determined by measuring the contact angle. Figure 1 shows the state of a water droplet on the surface of a solid.
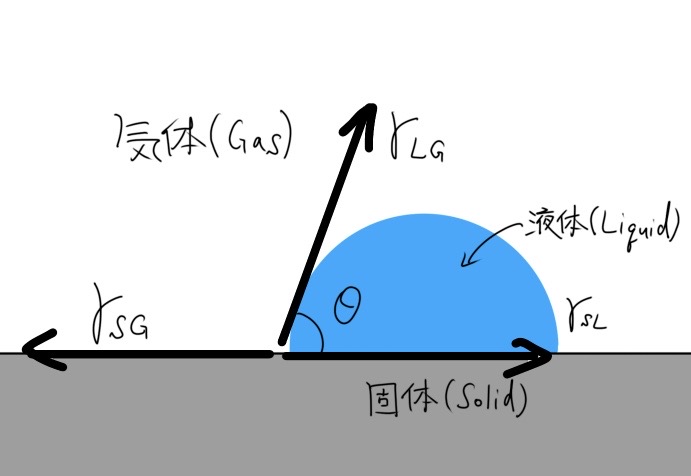
The contact angle is defined as “the angle θ at which the liquid/gas interface meets the solid surface”.
(What’s the interface? If you’re wondering, please click here. It is the boundary between the two substances.)
The equation that expresses the relationship between the contact angle and surface tension is
$$\gamma_\mathrm{SG} = \gamma_\mathrm{SL} + \gamma_\mathrm{LG}\cos\theta$$,
which is called Young’s equation.
Here,
$$\gamma_\mathrm{SG}, \gamma_\mathrm{SL}, \gamma_\mathrm{LG}, \theta$$
are the interfacial tension generated between Solid/Gas, Solid/Liquid, Liquid/Gas, and the contact angle, respectively.
In general, if the value of the contact angle θ is greater than 90 degrees, it is called hydrophobic. The so-called liquid becomes “rolling”. It is often observed on the surface of the leaves of plants. (Fig. 2)

Conversely, if it’s less than 90 degrees, it’s called hydrophilic. This is a condition in which a liquid soaks into a solid surface in part or in full. This is true for human skin as well.
In this way, wettability can be quantified. Next time, I’d like to expand on this Young equation a bit and dig deeper.
Sincerely,
The Principia-Lab Team
Reference
[1] Stimuli-Responsive Bioinspired Materials for Controllable
Liquid Manipulation: Principles, Fabrication, and
Applications.
[2] Drop Impact on a Solid Surface.
[3] Purity of the sacred lotus, or escape from contamination in biological surfaces.